
Want to stay on top of the science and politics driving biotech today? Sign up to get our biotech newsletter in your inbox.
Good morning. Today, we discuss how psychedelics companies are responding to the negative ad comm vote on Lykos’ therapy, results of Zepbound in MASH, and the fate of Novavax this fall.
Psychedelics companies look on the bright side
The day after a panel of FDA advisers rejected the use of MDMA-assisted therapy for post-traumatic stress disorder, shares in companies developing psychedelics dropped, while analysts said the decision could hinder the field’s ability to raise more funding.
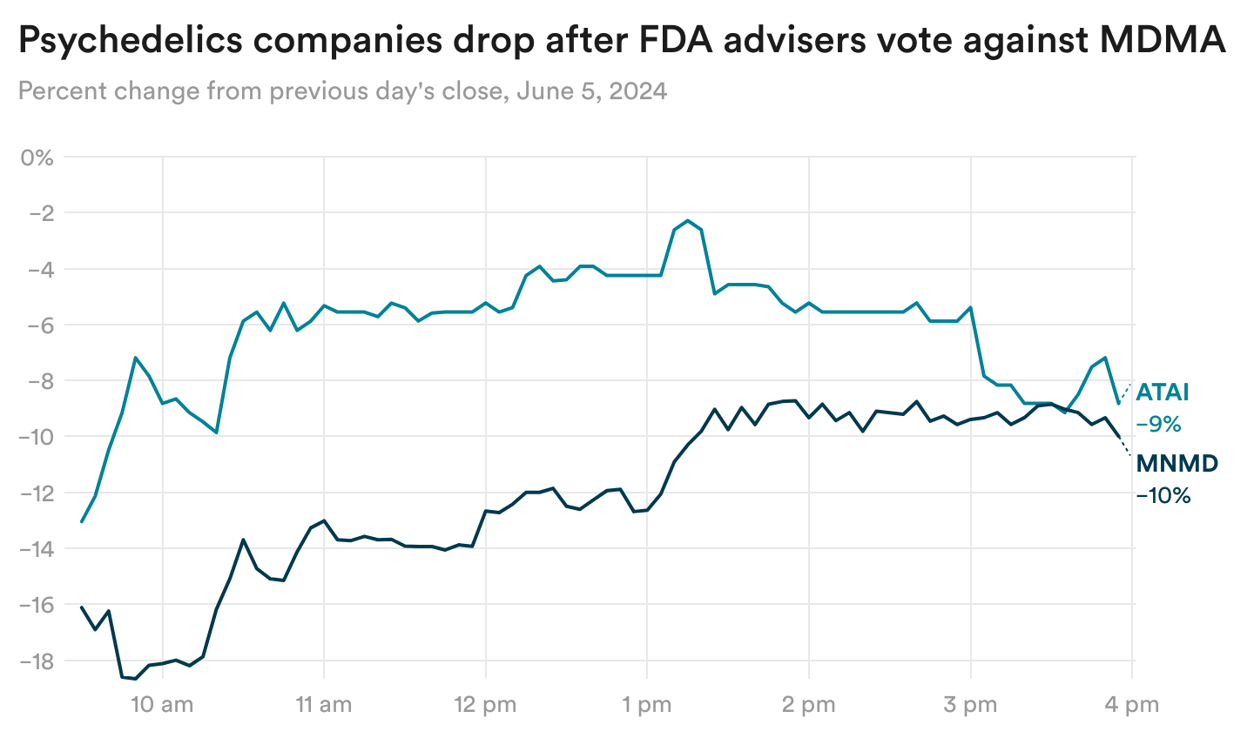
But, in interviews with STAT’s Meghana Keshavan, psychedelics companies said their optimism had not been dimmed — and that the concerns expressed about the approach used by Lykos Therapeutics provided a clearer blueprint for what it might take to get such a drug approved.
“It’s not the drug. It was the execution,” said Christian Angermayer, a financier who is deeply involved in the movement to turn long-shunned psychoactive substances, like the psilocybin derived from so-called magic mushrooms, into approved medications for depression and other mental illnesses.
Zepbound improved liver scarring in MASH patients
An abstract released yesterday showed that Lilly’s blockbuster obesity drug improved liver scarring, or fibrosis, in a Phase 2 MASH study. This was a key result observers were waiting for after Lilly in February reported positive results on MASH resolution from this study, but didn’t disclose exact data on fibrosis.
Analysts noted some caveats with the fibrosis data — there was a high proportion (30%) of patients on placebo who experienced fibrosis improvement, and there wasn’t a dose response among the cohorts taking the drug. Still, the differences between the treatments groups and placebo were statistically significant.
How much will obesity drugs like Zepbound ultimately affect the fate of liver-directed MASH therapies, though? Some analysts and doctors are expecting that obesity drugs may support use in moderate MASH patients, but severe MASH patients may end up benefitting more from liver-directed drugs.
Novavax will be able to supply Covid-19 vaccines this fall
From STAT’s Helen Branswell: The Food and Drug Administration’s vaccine advisory panel voted unanimously yesterday to recommend that this fall’s Covid-19 shots should be updated to target viruses in Omicron’s JN.1 lineage. That recommendation, if accepted by the FDA, is broad enough to ensure that Novavax, which has struggled to gain a substantial share of the Covid vaccine market, will be able to supply product to the U.S. this fall.
There was some discussion about whether it would be advisable to ask manufacturers to use a more current virus, KP.2 — an offshoot of JN.1 — in the fall vaccine. Both Pfizer and Moderna indicated they could produce either, and deliver their product in August. But Novavax, which has a longer production time than the messenger RNA vaccine manufacturers, said that if the target for this fall’s vaccine was KP.2, it would not be able to supply the U.S. for the 2024-25 vaccination campaign.
Most members of the committee seemed to feel that the differences between the two were not large enough to warrant preferentially recommending KP.2.
Antidepressant withdrawal symptoms may not be as frequent as once thought
A new systematic review found that about 15% of people experienced withdrawal symptoms after weaning from antidepressants, and in 2-3% of the cases, the symptoms were severe.
This analysis is “long overdue,” one external researcher says. While it confirms that withdrawal symptoms do happen with clinically relevant frequency, it also demonstrated a lower incidence than recent estimates based on online surveys. Those surveys generated public alarm when they suggested symptoms may occur in half or more the patients.
Read more from STAT’s Annalisa Merelli.
More reads
To submit a correction request, please visit our Contact Us page.











STAT encourages you to share your voice. We welcome your commentary, criticism, and expertise on our subscriber-only platform, STAT+ Connect